Fig. 8: Binding of Peptide Epitopes from Endogenous Viral Antigens to MHC-I Moleculesr
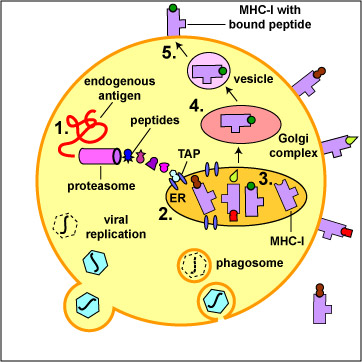

The body marks infected cells and tumor cells for destruction by placing peptide epitopes from these endogenous antigens on their surface by way of MHC-I molecules. Cytotoxic T-lymphocytes (CTLs) are then able to recognize peptide/MHC-I complexes by means of their T-cell receptors (TCRs) and CD8 molecules and kill the cells to which they bind.
1. Endogenous antigens, such as viral proteins, pass through proteasomes where they are degraded into a series of peptides.
2. The peptides are transported into the rough endoplasmic reticulum (ER) by a transporter protein called TAP.
3. The peptides then bind to the grooves of newly synthesized MHC-I molecules.
4. The endoplasmic reticulum transports the MHC-I molecules with bound peptides to the Golgi complex.
5. The Golgi complex, in turn, transports the MHC-I/peptide complexes by way of an exocytic vesicle to the cytoplasmic membrane where they become anchored. Here, the peptide and MHC-I/peptide complexes can be recognized by CTLs by way of TCRs and CD8 molecules having a complementary shape.
Last updated: Feb., 2021
Please send comments and inquiries to Dr.
Gary Kaiser